Strange chemistry may explain a seemingly impossible cloud in Titan’s upper atmosphere.
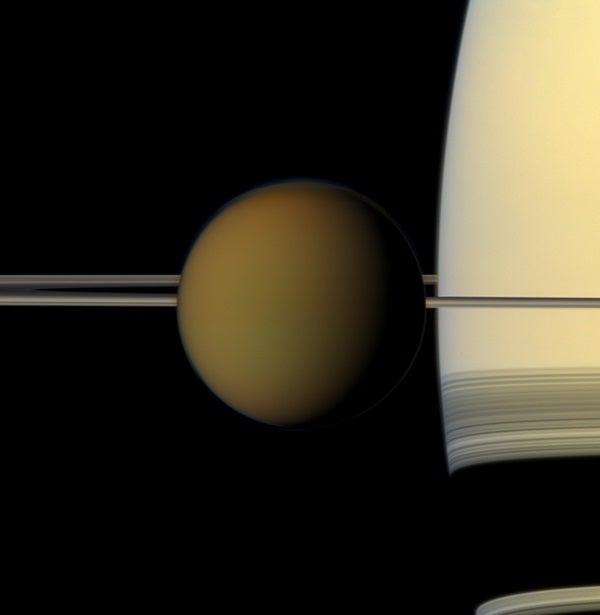
Scientists looking through Cassini’s 2010 data recently noticed something strange in an infrared snapshot of Titan’s stratosphere: a wide, thin, colorless cloud, about 20 km thick, high in the atmosphere above the moon’s north pole. The cloud was composed of frozen crystals of a compound called dicyanoacetylene, (C4N2), which everything we know about Titan suggests shouldn’t be there in any notable quantity.
Titan’s atmosphere is rich with complex organic chemistry, but this cloud was a rare sight.
“The dicyanoacetylene clouds are sparsely populated relative to many other organic compounds in the stratosphere that condense to form clouds, such as ethane, acetylene, cyanoacetylene, and hydrogen cyanide,” said Carrie Anderson of NASA’s Goddard Space Flight Research Center, co-investigator on Cassini’s Composite Infrared Spectrometer (CIRS) instrument
Mostly, that’s because there’s just not enough dicyanoacetylene vapor in Titan’s stratosphere to condense into ice particles and form a cloud. CIRS detected dicyanoacetylene vapor only about 1% dense enough to condense an icy cloud like the one CIRS observed in 2010. It looked like Cassini had sent home a picture of something utterly impossible.
That wasn’t the first time something seemingly impossible had appeared in Titan’s northern skies, though. Voyager 1’s spectrometer saw a similar dicyanoacetylene cloud on its 1980 flyby during Titan’s northern spring. Researchers were puzzled at the time, but concluded that the cloud was proof that dicyanoacetylene vapor had to be present in Titan’s stratosphere, and they just couldn’t see it. Voyager 1’s instruments, scientists decided, just weren’t sensitive enough to the right wavelengths of light to see the vapor .
Then, in 2010, again during Saturn’s northern spring, Cassini sent home a snapshot of a similar cloud. CIRS was sensitive enough to confirm that there wasn’t enough vapor to have produced it. Anderson and her colleagues spotted it during a recent analysis of the April 2010 data. The mystery was back.
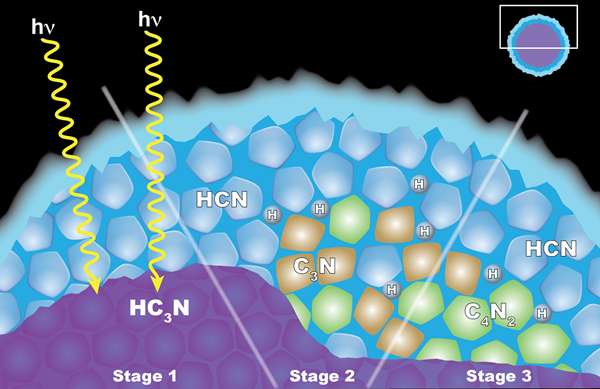
The cloud couldn’t have condensed from dicyanoacetylene vapor cooling and sinking over Titan’s north pole, but it had to form somehow. In a paper in the journal Geophysical Research Letters, Anderson and her team proposed a different idea. Instead of condensing from a mass of vapor, the dicyanoacetylene ice crystals are the product of a chemical reaction between ice crystals from two other compounds, triggered by ultraviolet (UV) rays.
About 150 km above Titan’s surface, cyanoacetylene (HC3N) vapor condenses into icy particles. As those particles sink another few kilometers through the stratosphere, they pick up a coating of hydrogen cyanide (HCN) ice particles. When UV rays strike the particles, they pierce that hydrogen cyanide shell and cause a chemical reaction inside. Dicyanoacetylene ice is a product of that reaction, along with hydrogen.
Cassini didn’t keep an eye on Titan’s northern latitudes long enough to watch the cloud disperse, but Anderson said, “Once formed, the ice particles will slowly precipitate downward in solid form, but it wouldn’t look like rain or snow. The cloud is very tenuous, and it’s in the stratosphere – high above the troposphere, which is the layer where rain clouds form.”
It’s similar to a process that happens high in the stratosphere above Earth’s poles. As water ice particles condense from the water vapor in Earth’s atmosphere, chlorine compounds attach to the ice crystals. UV light triggers a reaction between the chlorine compounds and the water, which produces nitric acid trihydrate (HNO3) and chlorine. The chlorine is part of the process that depletes ozone over the poles.
Testing the Model
These dicyanoacetylene clouds are rare, and they seem to be a seasonal phenomenon. “We think these stratospheric clouds form as polar winter gives way to early spring – when temperatures are quite cold but there’s enough sunlight, and other conditions also are right,” said Anderson.
According to CIRS data, the chemistry of Titan’s stratosphere does seem to cycle with the seasons. Late in the northern winter, cyanoacetylene is fairly abundant, but almost no dicyanoacetylene shows up in spectral readings. By early spring, though, the two compounds have switched places, with cyanoacetylene becoming scarce as dicyanoacetylene becomes more common. And that, say to Anderson and her colleagues, supports the idea that cyanoacetylene is being destroyed in chemical reactions that produce dicyanoacetylene as winter gives way to spring.
Meanwhile, in Anderson’s laboratory at Goddard, she’s irradiating mixtures of cyanoacetate and hydrogen cyanide ices with UV to see if they can recreate the reactions that produced Titan’s rare but mysterious dicyanoacetylene clouds. They’re also testing the idea in atmospheric chemistry models, which have been freshly updated with data from CIRS.
If models and laboratory experiments support Anderson’s idea, those findings could shed light on more than just one rare type of cloud in Titan’s upper atmosphere. As the researchers wrote in their paper, “it opens the door to investigating the production of other volatile ices in the atmospheres of Titan and the major planets, without the need for vapor condensation.”