Updated July 13, 8:48 a.m.
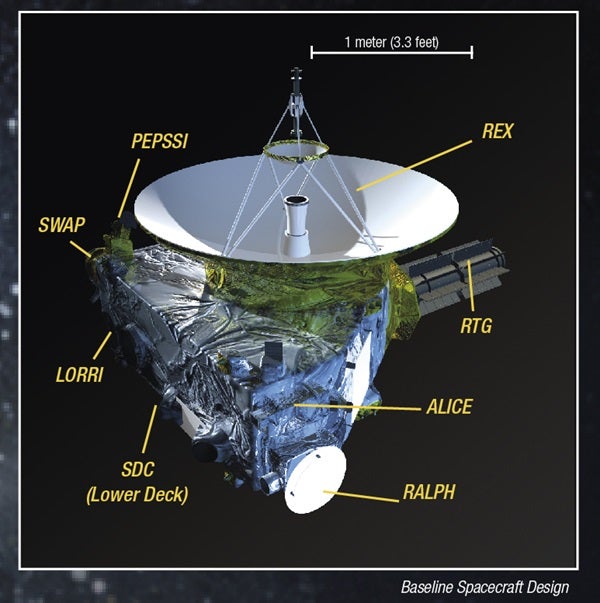
The New Horizons spacecraft has already cruised to the far reaches of the solar system, beyond the last full-fledged planet, and has entered the realm of the dwarfs. Like the outer solar system missions before it and unlike many space missions closer to home, New Horizons can’t rely on solar power so far from the Sun’s rays. So the Johns Hopkins Applied Physics Laboratory, who built the spacecraft, inserted a radioisotope thermoelectric generator (RTG), made by the Department of Energy (DOE), which uses heat from the decay of radioactive material to generate power for the spacecraft and all its instruments. Put simply, New Horizons runs on radioactivity. And in a pleasing twist of fate, the fuel that powers the mission to Pluto is none other than plutonium.
This technology is not new to New Horizons. Both Voyager missions, as well as Cassini, Galileo, the Mars Curiosity rover, and many more, use a similar RTG design, and it has proven its safety and reliability over decades of use and dozens of missions stretching back to the Apollo years. The specific material powering the RTGs is plutonium 238, a byproduct of the nuclear weapons production process.
Since the United States (and Russia) are now in de-proliferation mode, obtaining a steady supply of plutonium 238 has become a problem. The U.S. stopped production in 1988, and Russian sources have also evaporated, causing NASA’s stockpile to dwindle, especially since the material has a limited shelf life. In 2013, NASA began paying the DOE to produce plutonium 238 for them again, but it has been a difficult process to reboot, with small yearly yields, and concerns remain about the long-term supply chain. Plans to engineer a more efficient version of the RTG, which would have required only a quarter the amount of plutonium for the same power output, died on the drawing board when budget constraints kicked in in 2013. Part of NASA’s shrinking plutonium supply is slated for the Mars 2020 rover, and it will be a race over the coming decades between the DOE’s production rate and future space missions’ power needs. Despite the supply issues, radioactivity remains the best power source for deep-space missions.
So given that plutonium isn’t unique to New Horizons, is there any deeper meaning to be found in the fact that it powers NASA’s big Pluto trip? Not really. It’s simply fun to say. But while we’re on the subject of amusing plutonium trivia, let’s go back to the man who discovered the 94th element on the periodic table.
Actually, scratch that. Let’s start with the man who didn’t discover it.
In 1934, the famous scientist Enrico Fermi and his colleagues were bombarding the element uranium with neutrons in order to create heavier elements (known as transuranium elements, for their placement after uranium, the 92nd element on the periodic table). Fermi claimed to have produced both the 93rd and 94th elements, which he dubbed ausonium and hesperium. In one of science’s more frustrating dead ends, the German chemist and three-time Nobel Prize nominee Ida Noddack published a paper stating that in fact Fermi had done no such thing, and offered an alternative explanation for his results, an idea that would come to be known as nuclear fission. But without stronger evidence, her paper was largely ignored for four years, and Fermi’s interpretation assumed correct. It took Lise Meitner and colleagues providing a solid theoretical backbone for fission to disprove Fermi’s results.
In 1940, a scientist named Glenn T. Seaborg was working at the University of California, Berkeley, also bombarding uranium atoms to produce heavier elements. This experiment was successful. However, with World War II’s heightened security surrounding the clear military applications of such a discovery, the development was kept hush-hush until after the war. The journal Los Alamos Science relates the level of secrecy maintained by Seaborg, who, along with his colleagues, referred to element 94 by the code name “copper.” True copper had to become known as “honest-to-God copper!” around the lab.
By the time Seaborg and his team submitted a formal name, element 93, neptunium, was already christened as the first transuranium element. Following the obvious planetary pattern laid out for them, they submitted the name plutonium. For the element’s chemical symbol, Seaborg claimed in public lectures that he thought “Pl” was the most obvious option, but chose “Pu” as a joke, as its pronunciation sounds like a child complaining about a bad smell. But despite his expectations, no one ever called him on his gag, and the symbol remains.
Plutonium 238 may be a product of war, but it has by now more than proven its worth as a tool of exploration. And on Tuesday, it will do so one more time, in spectacular fashion.