Most people are busy with life on Earth. They rarely get the chance to think deeply about complex topics. And so they take the ideas of why we know certain things with relatively equal values — maybe these people are right, or maybe those people. I don’t know.
But the whole purpose of science is that we use analytical techniques that rely on the principles of the cosmos in order to find the correct answers to questions. One fundamental question in science is how we know the ages of what our planet is made of. How old are the rocks we hold in our hands? How old is Earth itself? For most of human history, no one really knew. Many thought Earth was extraordinarily old; many others thought it was 6,000 years old. But it is a critically important question in planetary science— a bedrock question (sorry for a terrible pun).
Radiometric dating is one way to know ages of rocks
So, how do we really know the ages of rocks, and of the planet? Scientists employ a technique called radiometric dating. It’s not a futuristic method to bring couples together. It’s a series of procedures that use important properties of elements in the cosmos to reveal secrets hidden within the rocks we often stand on. You see, virtually all rocks and other components of the planet we inhabit incorporate radioactive impurities in their makeup. Naturally occurring radioactive isotopes in structures act as clocks, as radioactive elements decay at precisely known rates. This technique was first developed in the early days of understanding radioactivity, by Bertram Boltwood, in 1907. Ever since, it has been the fundamental way we can reliably date most natural substances.

The details of how radioactive dating works are somewhat involved, and nine principal modern methods exist. But the basic story is that all matter is made of chemical elements and each element’s atomic number on the periodic table indicates the number of protons in its nucleus. Also, each element has a distinct number of neutrons in its nucleus. However, different isotopes of an element indicate differing numbers of neutrons. Particular numbers of isotopes of an element are called nuclides. The key is that some nuclides are inherently unstable and they will periodically undergo radioactive decay, transforming into another nuclide. The radioactive decay is governed by what scientists call the element’s half-life; after one half-life, one half of the atoms of the nuclide will have decayed. This decay can be measured with the nine methods, and therefore provide a radioactive clock to determine the ages of the substances they exist within.
Carbon-14 dating is good for things from the recent past
The modern dating methods offer a range of different approaches for researchers. The one most people hear about in news reports is radiocarbon dating, or carbon-14 dating. Carbon-14, a radioactive isotope of carbon, exists in all carbon-based living things. It is created continuously by the collisions of neutrons generated by cosmic rays entering Earth’s upper atmosphere. Plants acquire it by photosynthesis; we acquire it by eating plants and animals. The proportion of carbon-14 in the remains of an organism indicates the time elapsed since the organism died. This technique is most useful for bones and other remains of living creatures in relatively recent history. Carbon-14 has a half-life of 5,730 years. So it is really useful for remains of an organism that are no older than about 60,000 years, as by then the carbon-14 is almost entirely depleted.

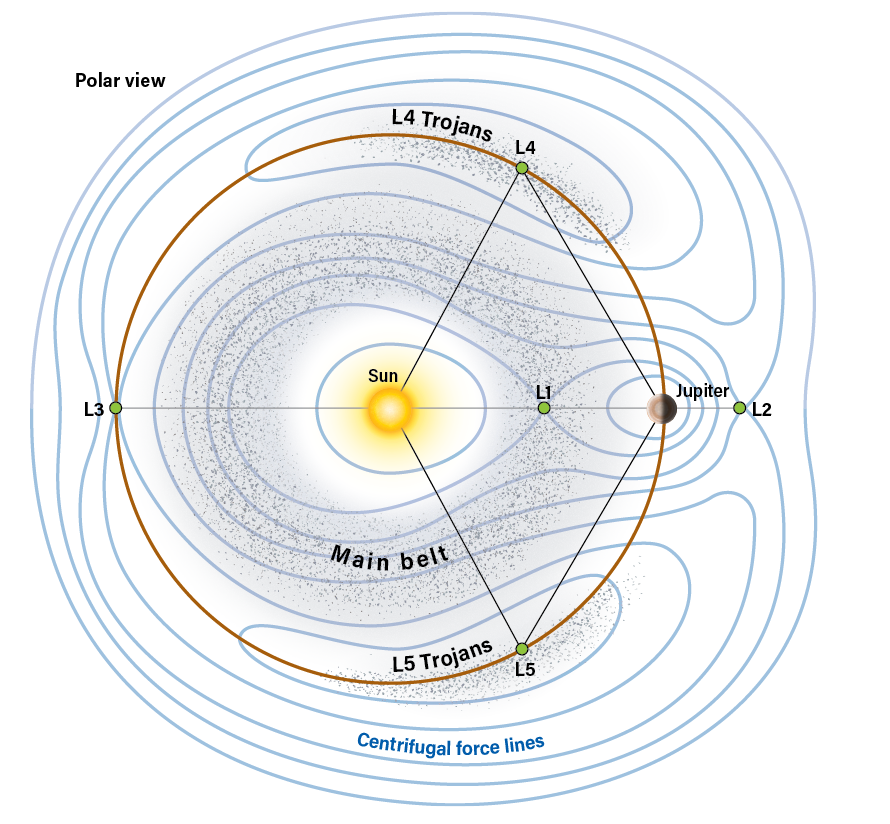
Other radiometric dating methods rely on the production of radioactive isotopes produced by nucleosynthesis in supernovae, however, and so provide clocks that extend vastly farther back in time. The uranium-lead dating method relies on uranium-235 or uranium-238 to date a substance’s absolute age. An abundant impurity in rocks exists in the mineral zircon, which almost universally contains small amounts of uranium.
Other methods also exist, and often act as cross-checks to obtain similar results through multiple approaches. There’s the samarium-neodymium dating method. This involves the decay of samarium into neodymium. Another technique is the potassium-argon dating method, which offers a half-life of potassium-40 of 1.3 billion years, and is helpful with v ages of rocks. The rubidium-strontium method is also helpful with very old specimens, as it features a half-life of 50 billion years.
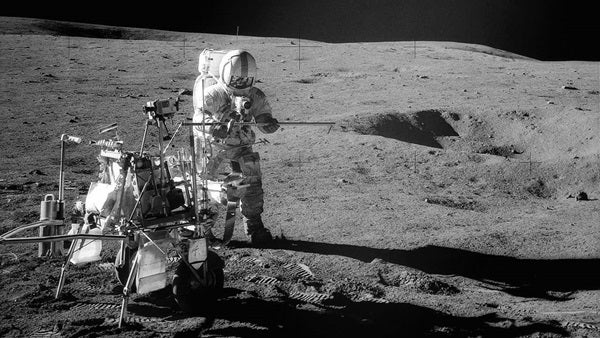
‘Big Bertha’ has been dated to more than 4 billion years old
By contrast, the uranium-thorium dating method offers a half-life of 80,000 years. Creatively, the fission-track dating method employs a polished slice of a material to examine the density of track markings created by the fission of uranium-238 impurities. The chlorine-36 dating method uses data acquired during underwater nuclear tests in the 1950s to date waters and ice of recent vintage. So-called luminescence dating methods are a bit different in that they measure the effects of background radiation on minerals.
As with all science, an ever-expanding sourcebook of experimental results provides a huge volume of information on the ages of rocks and other substances since 1907, the development of the first of these techniques. Because of radiometric dating, we know that Earth rocks are millions or billions of years old. We know that the ancient ideas of Earth’s relative youth are fantasies. We know that the oldest rocks on Earth, due to analysis of zircon crystals embedded within them, are 4.0 billion years old, and they lie in the Acasta Gneiss in the Canadian Shield. These rocks formed just about the time of the earliest emergence of life on Earth, and perhaps slightly before it, and some 600 million years after the formation of the solar system. Lunar sample 14321, “Big Bertha,” has been dated to an even older age, 4.46 billion years.
Scientific methods like radiometric dating are giving us amazing details of the true story of our solar system and our planet. They are unveiling a true story that is far more illuminating, and exciting, than any made-up fable of the past could hope to be.