Key Takeaways:
This article originally appeared in the December 2010 issue of Astronomy.
We — our Sun, Earth, and selves — are the products of everything that happened before our creation. The initial event, the Big Bang 13.7 billion years ago, still affects us today, not only in the resulting expansion of the universe, but also closer to home in the very fabric of the Sun. To figure out the whole story of where we came from and how things got to be the way they are, though, we must turn to the stars that made us — and what made them.
A reduced history of the universe
In the beginning, there was hydrogen. Just 3 minutes after the Big Bang, hydrogen atoms — protons — and neutrons collided to create helium and a little lithium.
So it would have remained, but for the stars that condensed from the expanding milieu. The first stars were massive. Supporting themselves with nuclear fusion in their deep, hot cores, they transformed hydrogen into helium, then helium into carbon and oxygen, and these into yet heavier elements. When they got to iron — the end of the fusion line — they blew up as the first supernovae (technically classified as “type II” supernovae). In the explosion’s cauldron, more fusion and other processes created nearly all of the periodic table.
In the explosion’s cauldron, more fusion and other processes created nearly all of the periodic table. The detonations scattered these new elements into the interstellar gases from which the next generation of stars would form.
Dust from the explosions cooled the interstellar gases, which allowed for stars with a greater range of masses. The more massive ones again blew up, while those of lower mass became the more ordinary stars we still see today. When their fusing interiors stopped at carbon and oxygen, they shrugged off their outer atmosphere to expose their cores as the first white dwarfs. A few of those within close binary systems grabbed enough mass from their companions to exceed the white dwarf limit of 1.4 solar masses and again blew up as another kind of supernova (“type Ia”).
Both kinds of supernovae sent ever more heavy stuff into space, and elements made inside the giants through more leisurely neutron capture were broadcast through their mighty winds.
Each generation of stars contains more carbon, oxygen, iron, and zinc. Elements beyond hydrogen therefore steadily build up as more stars create them, and their different origins dictate different growth rates for each element.
Then, 4.6 billion years ago, came a galactic milestone, at least for us: The Sun’s birth, bringing with it some 9 billion years worth of previous element formation.
Astronomers can therefore look back in time using the Sun, which contains a record of the workings of the universe before it and Earth were born. The chemical composition of the star thus becomes a fundamental data point with which to test broad theories of stellar and galactic evolution. But how can we learn the chemistry of a fearsomely hot body 93 million miles (150 million kilometers) away? After all, we can’t just send a satellite to “bite off ” some and bring it home.
Meanwhile, back in the lab
Actually, it turns out we can, if we let nature do it for us. The inner planets and asteroids also carry the Sun’s record of heavy elements. Earth won’t serve because its surface has undergone numerous modifications, and the other planets are currently inaccessible. But the most ancient meteorites, originally from the asteroid belt, which have changed least since birth, will work — at least in part.
Astronomers take these pieces of the primitive solar birth cloud that have landed on Earth and bring them into the lab for precise analysis. Although we learn much about them and the solar system that created them, they have unfortunately lost — or never had — the lightest, most volatile elements. We want to compare abundances with hydrogen, but meteorites have none (except within their small amounts of water). Nor do they have helium with which to test the amount produced by the Big Bang. Nor is there much of any other noble gas such as neon or argon. To learn these details, we must turn to the Sun — and to the very heart of solar studies, the solar spectrum.
Show your colors
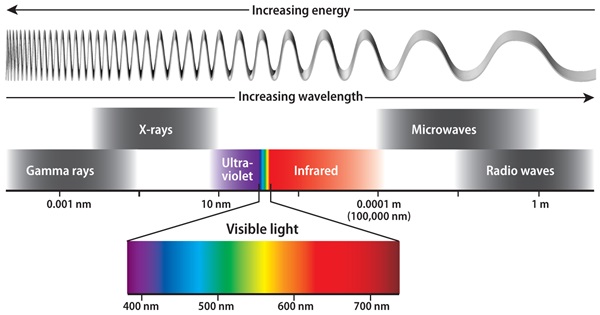
Light, which moves at 186,000 miles (300,000 km) per second in a vacuum, behaves as both a wave and a particle (called a photon) at the same time. The length of the wave — that is, the physical distance between wave crests — determines the color we see. In the visual spectrum, longer wavelengths of 700 billionths of a meter (nanometers) appear red, short ones of 400 nm appear violet, and all the other colors fall in between. Past the long side is the infrared, and past the short lies the ultraviolet. The amount of energy the wave/photon carries depends on the wavelength; violet light is almost twice as energetic as red, and ultraviolet proves downright dangerous (as anyone who’s ever gotten a sunburn can attest).
From the light of the Sun comes a smooth rainbow of colors, their individual richness dependent on the average solar surface temperature of 5780 kelvins (9900° Fahrenheit). Spread the spectrum with a prism, and it breaks into hundreds, even thousands, of segments separated by dark gaps, or “lines.” Each line is the result of a particular chemical element in the outer solar gases that absorbs the light from the continuous, unbroken spectrum the Sun generates.
Journey from darkness
The amount of light removed from the solar spectrum by the absorption line — its “strength” — depends ultimately on the abundance of its parent element. But how do we determine that?
It’s a tough job, as the number of steps is rather large. The strength of the line depends on two things: the number of atoms with electrons suited to absorb that color of light (the orbital “population”), and the likelihood that such an atom actually absorbs that light (the “transition probability,” determined through lab experiments or calculation). Using theory and further experiments, we then relate the population (derived from the line strength) to the total number of atoms to get the elemental abundance.
All that effort requires a good model of the Sun. And therein lies the real problem. Absorption lines have width to them and thus, plotted on a graph of darkness against wavelength, a “profile.” The Sun is not a hard ball at a specific, uniform temperature. What we see as the solar surface is actually a partially transparent layer — the “photosphere” — in which the temperature continuously changes from bottom to top. Most absorption lines are made at slightly different layers, and so at different temperatures. Indeed, each part of the absorption profile of a line is formed in a different layer at a different temperature. To relate line strength to the number of atoms producing it, we need to know how temperature relates to photospheric depth: the “temperature gradient.”
To an extent, scientists can determine the temperature gradient using solar limb darkening. Because the Sun is a sphere, your line of sight at the edge, or limb, does not penetrate as deeply as when you look at the center. You therefore see cooler gases, and the solar surface looks dimmer and redder. We can also use models, which depend on calculations of the opaqueness of the gas. These in turn depend on, naturally, abundances.
To LTE or not to LTE
The amount of calculation involved in modeling the Sun is daunting. With no powerful computers, early modelers had to be content with simplicity, so they assumed the solar photosphere would be quiet and layered with smooth changes. Moreover, the solar gases were assumed to be in simple “local thermodynamic equilibrium” (LTE), in which each atomic process, whether absorption or collision, was exactly balanced by the opposite process.
The final results were reasonably close with amounts from meteorites, which use the equivalent solar values of silicon as a benchmark to fill in any blanks. And there are many. The lightweight element lithium (number 3) is extremely scarce in the solar photosphere, and there are no solar abundances for such well-known elements as iodine, platinum, mercury, and uranium (not to mention obscure ones like tellurium, tantalum, and rhenium).
But given advances in computing power and the quest for better absorption probabilities, we can improve the model, making it more complex so it can fit the actual solar conditions. The first to go was the assumption of LTE. Too many options exist for atomic processes to assume constant balance. To calculate the population of a given electron orbit therefore requires that all atomic processes and orbital interactions be taken into account, which significantly boosts the amount of calculation.
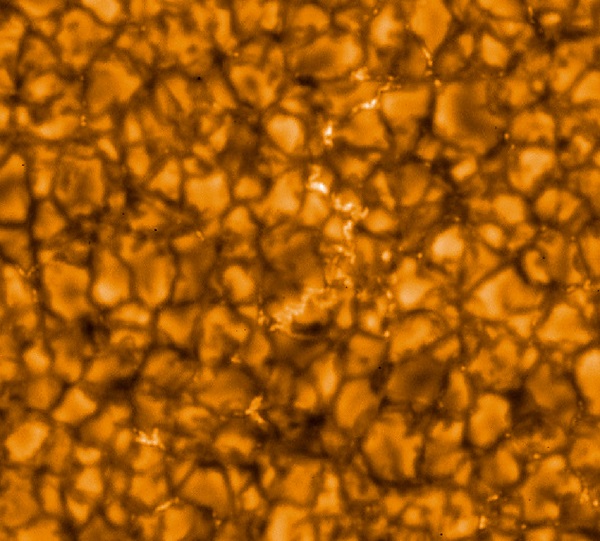
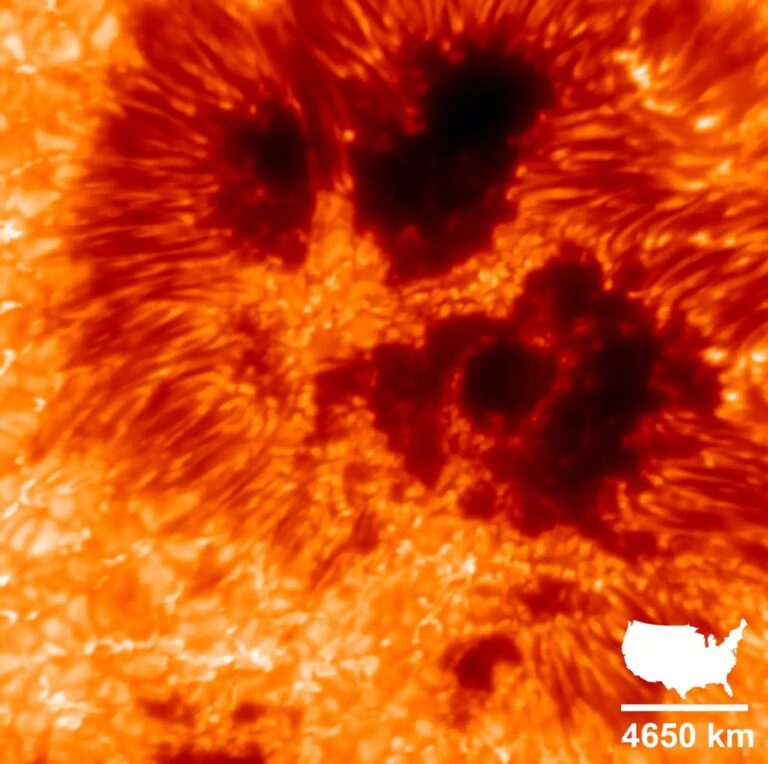
And then, have you ever actually seen the surface of the Sun? The photosphere is constantly in a roiling state of convection, with hot gases rising and cool ones falling, each in a cell the size of Texas. Temperatures depend on whether you look at the center of a cell, near an edge, or at a dark falling zone at the edge. (And don’t even think about sunspots.)
With the advent of powerful supercomputers, the latest developments involve 3-D models, in which the reality of convective motion, as well as non-LTE interactions, are taken into account. Ideally, each generation of model is more successful and realistic than the one that came before.
The envelope, please
Even the simple models, however, when coupled with meteorite results, reveal some remarkable abundance patterns. Helium (determined through analysis of solar vibrations) comes in at 8.5 percent as abundant as hydrogen, which, given a bit of enrichment by previous stellar generations, fits with predictions made based off the Big Bang. Beyond that, the next most abundant element is oxygen (about 0.05 percent as abundant as hydrogen), followed closely by carbon, neon (obtained from the solar corona and wind), and nitrogen. All fit with what one would expect from current theories.
The light elements in between — lithium, beryllium, and boron — have low concentrations, which is no surprise because the stellar fusion process skips over them. Except for minimal Big Bang production, scientists now know these elements are produced in collisions between cosmic rays (high-speed particles) and interstellar atoms. Lithium is especially scarce in the Sun because convection pushes it downward toward the core where nuclear reactions destroy it (so the amount of Lithium provides a handy way to measure solar age).
Except for big dips at fluorine and scandium, elements after the carbonnitrogen- oxygen (CNO) group see a general decline, with a final rise around iron, which makes sense given that the element is the end of energy-producing fusion. We then see a steep drop as we move to the elements created by neutron capture, whose abundances decline quickly with increasing proton number, but with rises around barium and lead. Superimposed on the whole abundance pattern is a jagged sawtooth shape in which evennumbered atoms are somewhat more plentiful than their odd-numbered neighbors, the result of a preference for paired protons in atomic nuclei.
So who cares?
The results matter to just about everybody. The solar chemical composition sets the example by which other stars, our solar system, and even Earth are understood. It thus touches the very heart of our origins. Galactic evolution modelers use it to test their predictions, as do astrophysicists interested in nucleosynthesis — the making of heavy atoms from light ones. Interstellar astronomers use solar abundances to determine how often heavier atoms (considered “metals” by astronomers) condense on dust grains, which results in metal-depletion of the gas and which is important in understanding star formation. Deviations between solar abundances and cosmic-ray data reveal the interactions between these ultra-fast particles and the interstellar medium that produced the Sun, which tells us more about each. Of major importance, solar abundances are vital for calculating the cloudiness of solar and stellar gases, upon which theories of structure and evolution depend heavily.
The solar composition is crucial in understanding how the Sun — the basis for life — works. The new 3-D models drop the CNO abundances by 70 to 80 percent, which fits with results from other stars and with diffuse and local planetary nebulae. Yet not all is well. Solar oscillations allow the determination of the speed of sound within the solar interior, which depends on chemical composition. The calculations of the expected speed using the results of new 3-D models give notably poorer agreement with the observed speeds than do earlier, simpler models.
Moreover, the depth of the convection zone calculated under the new abundances is less than that found from the oscillations. Nobody knows what’s wrong. Either the models need improving, or the solar oscillation folks have some work to do. Or maybe both. However it works out, from its chemistry we’ll learn more about the Sun and the stars that surround us, and thus of the universe of which we are all a part.