Key Takeaways:
- Planck's constant reveals the quantum world's fundamental energy levels.
- It shows how light and matter exist in discrete units (quanta).
- This constant defines limits to our knowledge of particle properties (Heisenberg's Uncertainty Principle).
- It governs the behavior of atoms and subatomic particles.
Planck’s constant is one of the most important numbers in all of physics. It is, essentially, the ultimate guide to the quantum world. It tells us where quantum effects are important, the fundamental energy carried by light and matter, and more. And it all started as an ugly hack.
The black body
In the late 1800s, physicists were struggling with one of the most challenging problems of the day. It involved what is called a black body, which was an experimental device consisting of an empty shell painted black on its inside walls (hence the name). When heated, the walls of the device emitted light with very particular characteristics — a “spectrum” whose intensity peaked at a specific wavelength of light and diminished from there.
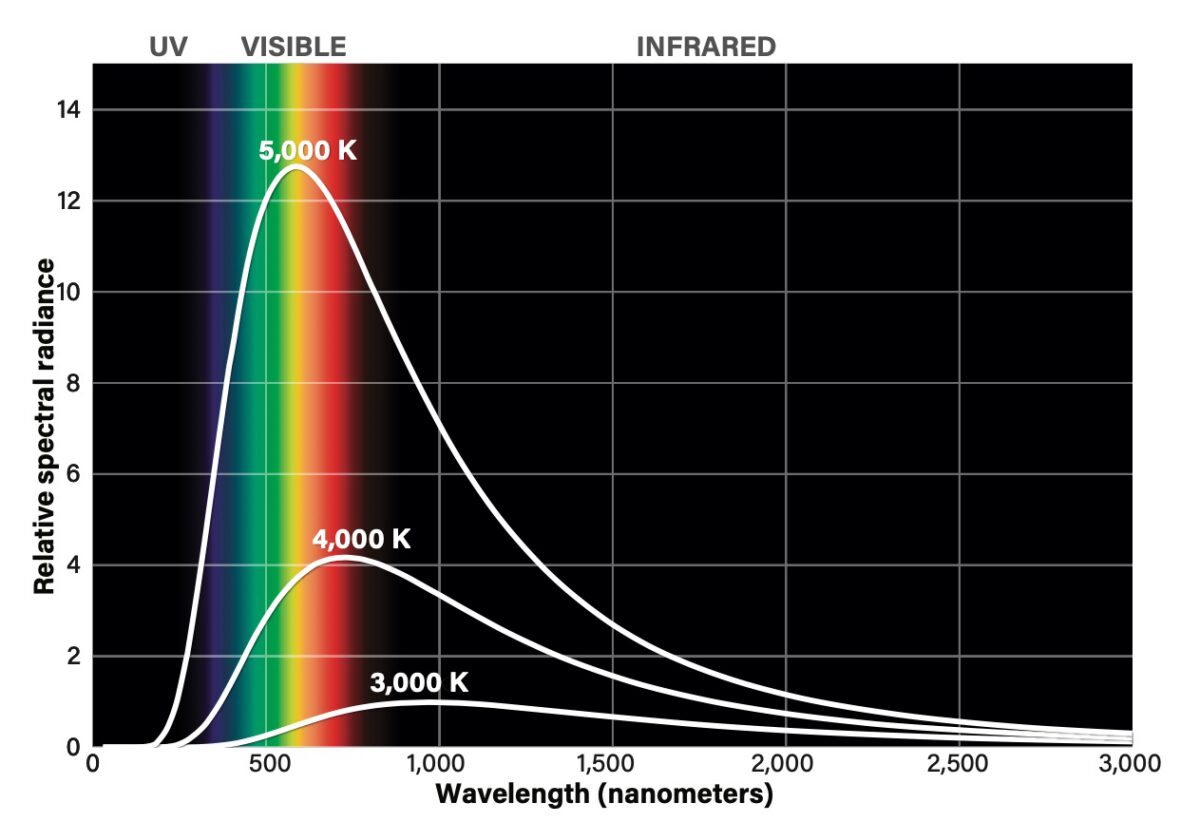
Despite attempts from the leading thinkers of the age, nobody could derive a formula to explain this so-called black body radiation. And then Max Planck came up with an idea — but he wasn’t exactly fond of it.
In 1899, he introduced what he considered a “mathematical trick.” He assumed that the walls of the black body could only emit radiation in specific chunks, with the size of these chunks dictated by a specific number. The trick worked, and it was the world’s first introduction to the realm of the quantum. That number, about 6.62 x 10-34 joules per second, is now known as Planck’s constant, and often written as the letter h.
In 1905, Albert Einstein dropped another bombshell. He argued that it wasn’t just the emission or absorption of radiation that came in discrete chunks. It was light itself. In other words, there was a smallest possible amount of light, which we now call the photon.
Planck’s constant tells us how “big” a photon is. If you have light of a given frequency, Planck’s constant tells us how much energy that light has, and vice-versa.
Quantum effects
As the field of quantum mechanics emerged in the early 20th century, Planck’s constant appeared again and again. For example, it’s not just light that’s quantized, but also motion. In the macroscopic (everyday) world, it appears to us that angular momentum can have any value — anything can spin as quickly or as slowly as it pleases. But experiment after experiment revealed that this is not the case. Angular momentum can only appear as a fixed multiple of Planck’s constant: either one or two or a million or a trillion, but never in half-steps or fractional increments.
We never notice this in everyday life because Planck’s constant is so small, and quantum effects only become important at incredibly tiny scales. But at those tiny scales, Planck’s constant shows up everywhere.
The constant also determines the wavelength of matter waves. Any object, including you, is not really a pointlike particle. At the heart of quantum mechanics sits wave-particle duality, where all objects exhibit properties of both points (highly localized in space with definite position) and waves (spread out over space). Planck’s constant gives us the size of the wavelengths associated with matter. Again, macroscopic objects like you and me have only very, very small wavelengths, while subatomic particles like electrons have relatively large waves.
Planck’s constant also plays a major role in Heisenberg’s uncertainty principle. This principle states that it is impossible to know both the position and momentum of any particle with infinite precision. In fact, the more you know about one of those properties, the less you know about the other. This seesawing of knowledge is governed by Planck’s constant — it tells us the limit of what we can know.
All of atomic physics rests on Planck’s constant. Electrons, which live outside of atomic nuclei, cannot have any position they want. Instead, the orbits are quantized, meaning that — once again — they are restricted to very specific distances from the nucleus. The location of these orbits and the spacings between them are determined by Planck’s constant. This means that atoms can only emit or absorb specific frequencies of light: the frequencies that match up to the right energy levels as electrons jump from one orbit to another.
Related: How do scientists determine the chemical compositions of the planets and stars?
A new realm
Planck’s constant is our signpost that we have entered the quantum world. If the property of any system — whether it’s energy, size, or duration — approaches Planck’s constant, then we know we have to leave the familiar world of macroscopic physics behind and take into account quantum effects.
But why Planck’s constant has the value that it does is a major outstanding mystery in physics. The value of the constant cannot be derived from any theory of physics. All we know is that at incredibly small scales, the universe operates according to unfamiliar rules, and that Planck’s constant is in charge.