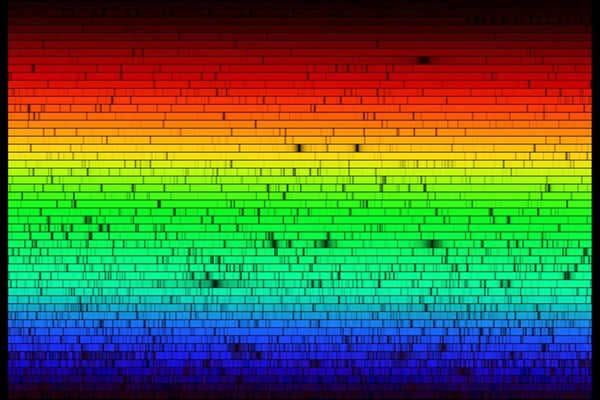
Q: How do scientists know what spectral lines belong to which compound?
A: Each atom and molecule has its own light fingerprint that, like yours, is unique. But unlike yours, this fingerprint is made of light. Elements and compounds emit identifying sets of “colors,” or wavelengths, of light. (“Colors” is in quotes because the light is not always visible, extending to infrared and radio bands on one side and ultraviolet and gamma rays on the other.) No two color combinations are the same, allowing astronomers to accuse specific chemicals of being in stars, gas clouds, or planetary atmospheres.
But how did astronomers get these chemicals’ fingerprints in the first place? Just like in a crime drama, they brought the atoms downtown (to the station). And by “station,” I mean laboratory. There, chemists put the atoms through all kinds of trials, where they vary the temperature, collect the light that results, and precisely determine the different wavelengths that make that light up. Once the fingerprints are “in the system,” astronomers can go look for matching sets in space.
It may sound simple, but consider this: Few things in the universe are made of one pure substance. Astrochemists, as those who work in this field are called, have to separate the signature of hydrogen from the signature of helium from the signature of ethylmethylamine, which is like trying to determine what a person’s fingerprint looks like when 10 other suspects’ prints are on top of it.
Associate Editor