Many space-lovers know the phrase “We are all made of star stuff.” And it’s true — our planet formed from the dust cloud left over from the formation of our Sun, and from the planet, all life. That cloud, in turn, was born from the remnants of past generations of stars. But the elements that stars produce over the course of their lives are just one part of the story. The rest of the story — involving stars’ apocalyptic deaths — is even more exciting.
Stellar factories
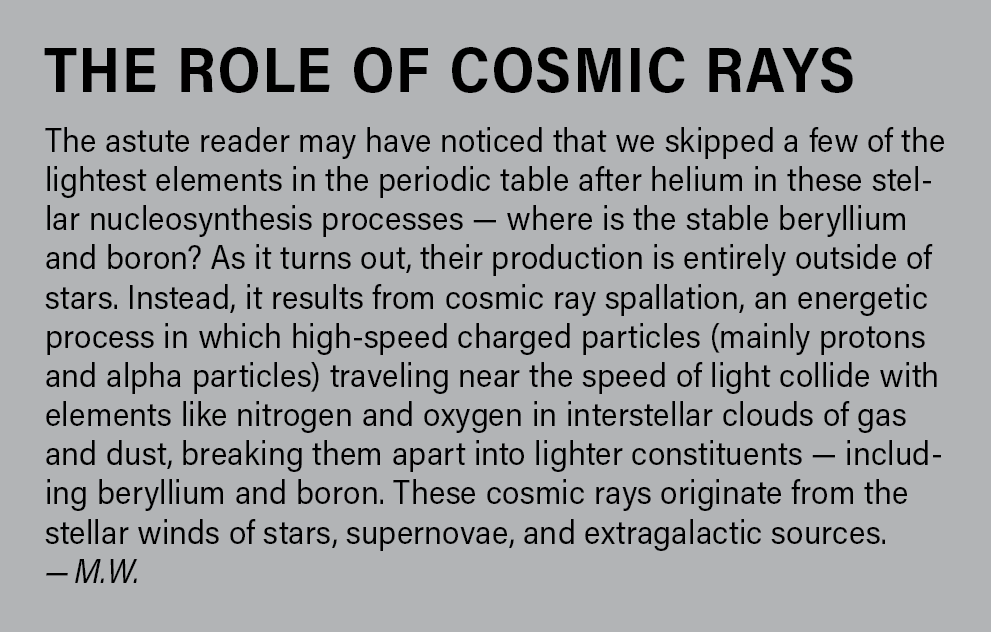
Our story begins as all stories do, at the beginning — in this case, the very beginning: the Big Bang. As the nascent universe rapidly expanded, it also cooled. After the first few minutes, it was cool enough for protons and neutrons to combine into atomic nuclei. During this phase, it was still hot enough for fusion to occur freely, so hydrogen (one proton), helium (two protons and two neutrons), and a small amount of lithium-7 (three protons and four neutrons) — the first three elements on the periodic table — could form. This process of creating the first elements is called primordial nucleosynthesis.
But after about 20 minutes, the 300-light-year-wide universe became too cool for fusion to continue. It would be another 370,000 years before it cooled enough for electrons to finish bonding with nuclei and form electrically neutral atoms, ending a period known as recombination. At this point, the temperature of the universe was approximately 4,000 kelvins (a little cooler than the surface of the Sun) and 84 million light-years across. Once the electrons were no longer free, light could pass through the cosmos unhindered: This was the genesis of the famed cosmic microwave background, the earliest surviving photons that we can detect.
After that, at least 100 million years passed before the first stars began to form, marking an end to the period that cosmologists call the Dark Ages. The exact timing of when the first stars formed is an unanswered question that the James Webb Space Telescope is exploring.
Stars exist in a delicate balance of the inward-pulling force of gravity versus the outward-pushing energy from fusion. As stellar material is pulled inward and compressed, it heats up, and eventually it becomes hot enough for hydrogen fusion to occur.
Now, the cores of stars are not actually hot enough to force positively-charged protons to come near enough to fuse — they naturally repel each other, like trying to push the same ends of two magnets together. Instead, a fascinating effect known as quantum tunneling must occur, where if two protons collide with each other enough times, there is a tiny chance they will eventually combine. While the probability is minuscule, if you have many such collisions occurring (as in the very dense cores of stars), eventually some fusions are bound to happen.
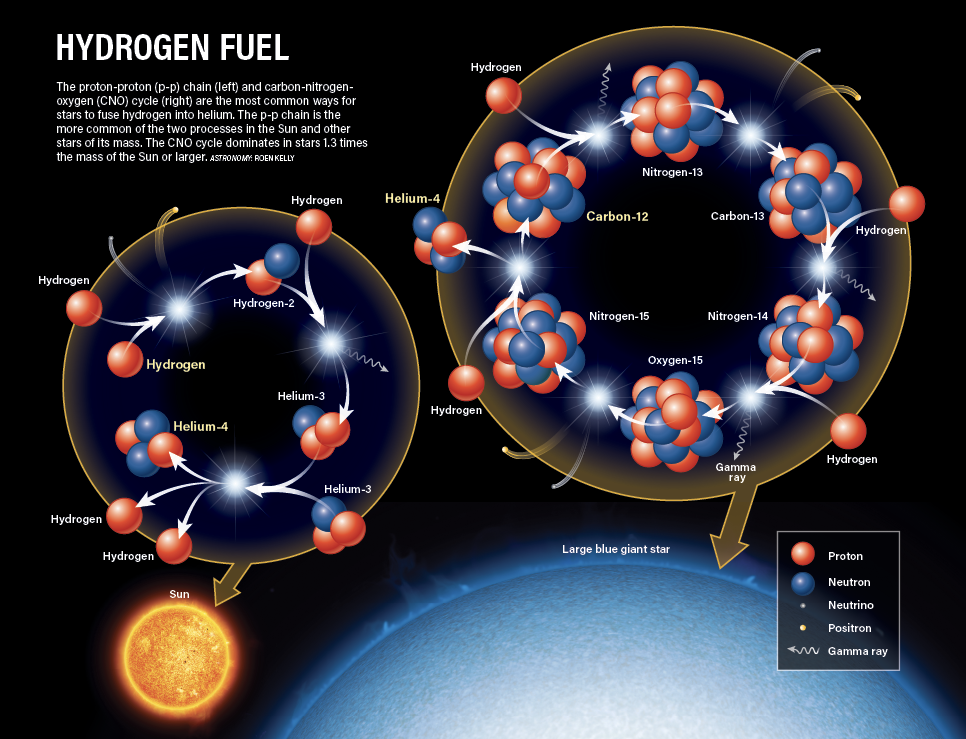
The product of hydrogen fusion, a helium nucleus, has four particles: two protons and two neutrons. So, two hydrogen nuclei (each just one proton) do not directly become helium; they must go through a few steps. In the most common stellar fusion process, called the proton-proton chain, one of the protons transforms into a neutron through a process called beta decay, which then combines with another hydrogen to become helium-3 (3He). Finally, two 3He nuclei combine to produce helium-4 (4He), with two hydrogen nuclei left over that can go on to other fusion reactions. Each step releases energy in various forms, including gamma rays (high-energy light well outside the visible spectrum), neutrinos, and positrons. The gamma radiation counteracts the force of gravity and keeps the star from collapsing under its own weight, existing in a balanced state called hydrostatic equilibrium.
Eventually, stars exhaust their stores of hydrogen to burn and, unable to resist the pull of gravity, the core begins to collapse inward. This causes the temperature to rise even more, and in stars at least 1.3 times the mass of our Sun, it becomes hot enough for helium fusion to start, restoring the balance. The core temperature reaches 100 million kelvins, six times that of the Sun. Two 4He nuclei, also referred to as alpha particles, can now fuse to beryllium-8 (8Be), but unfortunately, this is extremely unstable and almost immediately falls apart, back into the two helium atoms.
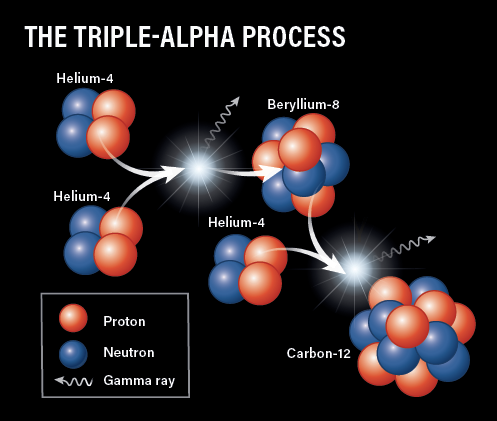
But, if a third 4He nucleus comes along in the tiny fraction of a second before the 8Be splits, they can form stable carbon-12 (12C) in the so-called triple-alpha process. Eventually, with enough 12C around, these heavier stars can produce additional energy through what’s known as the carbon-nitrogen-oxygen (CNO) cycle. In this cycle, a series of proton captures and beta decays take that 12C through a few different forms of carbon, nitrogen, and oxygen back around to 12C, ready to start the process all over again. Energy in the form of gamma rays, neutrinos, and positrons, is produced at each step.
In addition, 12C can fuse with 4He, making oxygen-16, which can itself fuse with another 4He to make neon-20; this process continues up the table in steps of two protons, skipping every other element in the periodic table, until iron-56 and unstable nickel-56 are reached. At that point, energy can no longer be released from fusion; instead, fusion would steal energy.
About half of the remaining stable elements heavier than iron are created through the s-process in stars (s for slow), particularly in asymptotic giant branch (AGB) stars, a late-life stage of stars with low to medium mass. In this process, an atomic nucleus captures a free-roaming neutron produced in the CNO cycle to add to its collection. If that number of protons and neutrons is stable, then it can go on to capture another; if not, then one of the neutrons will beta decay into a proton, walking the atom one step up the periodic table. This process accounts for much of the production of elements such as strontium, zirconium, barium, mercury, and others. However, this process can only take us as high as bismuth, since it is too slow to continue up to the less stable heavier elements.
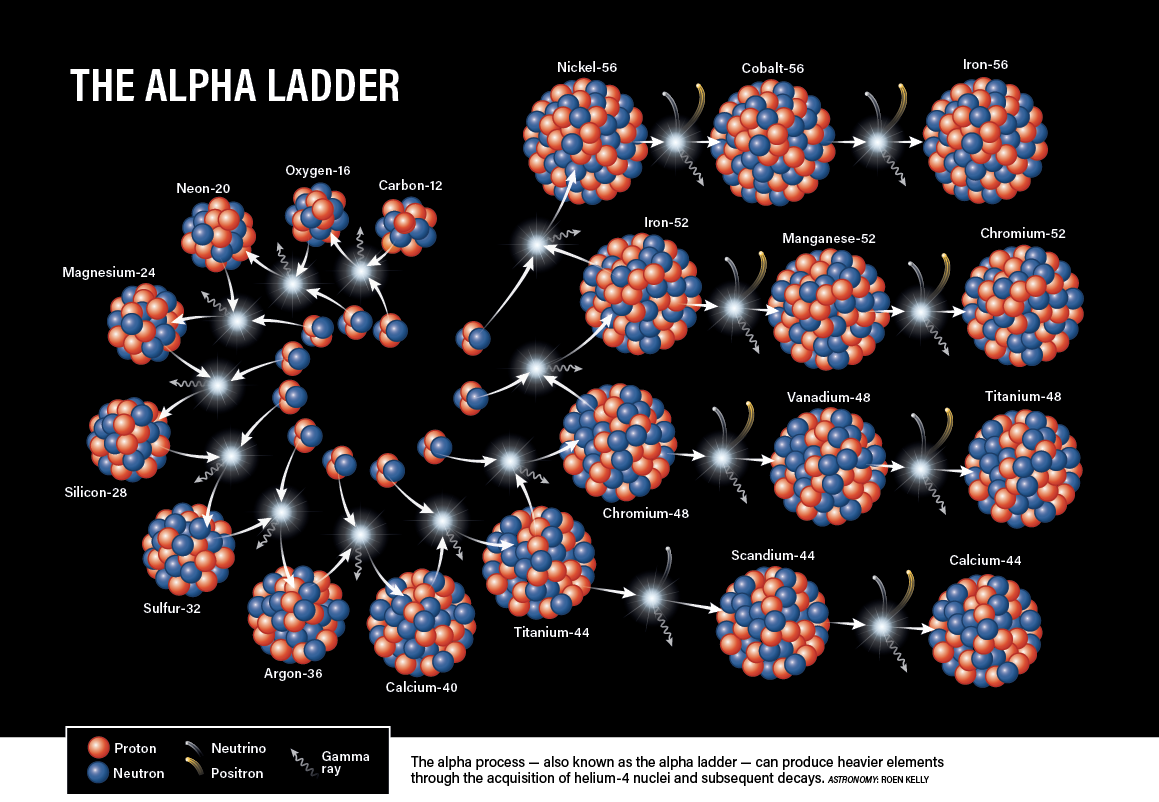
The rest of the story
The rest of the elements, including ones skipped over during stellar fusion, result in one way or another from explosions of stars. During a core-collapse supernova in stars more than eight times the mass of the Sun, the gravitational collapse produces a fast-moving shockwave and the temperature spikes to some 100 billion kelvins. This kickstarts an explosive round of fusion that generates a healthy portion of the elements between neon and nickel.
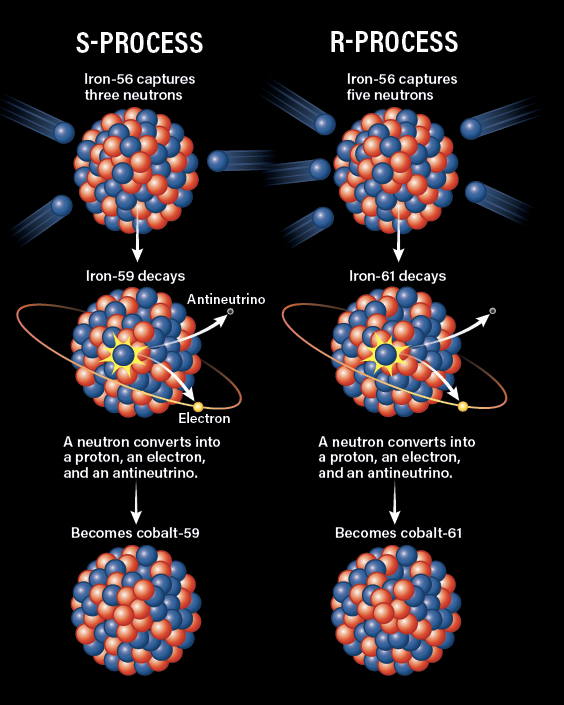
After the supernova, for stars that are not massive enough to collapse into a black hole, the compact core that remains becomes a neutron star. In this extremely dense remnant, protons and electrons from the stellar core are compressed so tightly that they combine through a process called electron capture, transforming into neutrons. The neutron-rich environment enables the r-process, a significantly faster (i.e., rapid) version of the s-process. In the r-process, nuclei keep capturing neutrons before they have time to beta decay until the nucleus becomes too unstable to stay together. Then the nucleus undergoes a series of beta decays, stepping up the periodic table, until it is more stable.
The r-process, which can occur in both supernovae and violent neutron star disruptions, is responsible for much of the rest of the elements heavier than iron, including the long-lived, naturally occurring radioactive elements beyond lead. It is also entirely responsible for elements such as uranium and thorium.
But how do these heavier elements eventually wind up in planetary systems? It was long thought that most of the r-process elements were produced during supernovae and expelled out into space, but it was later discovered that these elements are 100,000 times less abundant in supernovae than they should be. So where are they coming from?
In 2017, the first neutron star merger was observed by the LIGO and Virgo gravitational-wave observatories, and the answer became clear: A far greater amount of r-process elements are ejected when neutron stars collide with each other (or sometimes with a black hole) than are formed during a supernova. Two neutron stars can form near each other when a pair of stars orbiting together both end their lives in a core-collapse supernova.
That might sound like a rare event, but since LIGO came online in 2015, it has already detected two neutron star mergers, meaning that the phenomenon must be relatively common. Our galaxy is estimated to be home to some 1 billion neutron stars, and these mergers are thought to occur on average every 10,000 to 100,000 years; a seemingly long period of time for us, but only a blip on the cosmological timescale. The material ejected from neutron star mergers eventually ends up in newborn planetary systems, including our own about 4.6 billion years ago.
When someone says we are made of star stuff, it’s more than just the humdrum daily fusion inside ordinary stars — the calcium in our bones and the iron in our blood come from enormous supernova explosions, while the gold in our wedding rings and the heavy elements in Earth’s crust come from violent mergers of unimaginably dense neutron stars.
So, we can trace our cosmic roots all the way back to the beginning. The hydrogen that formed in the first few minutes of the universe’s existence was fused in stars and transformed in stellar explosions to become every other element that exists, eventually finding its way into the formation of Earth and the emergence of life. We really are made of star stuff — and in more exciting ways than you think!