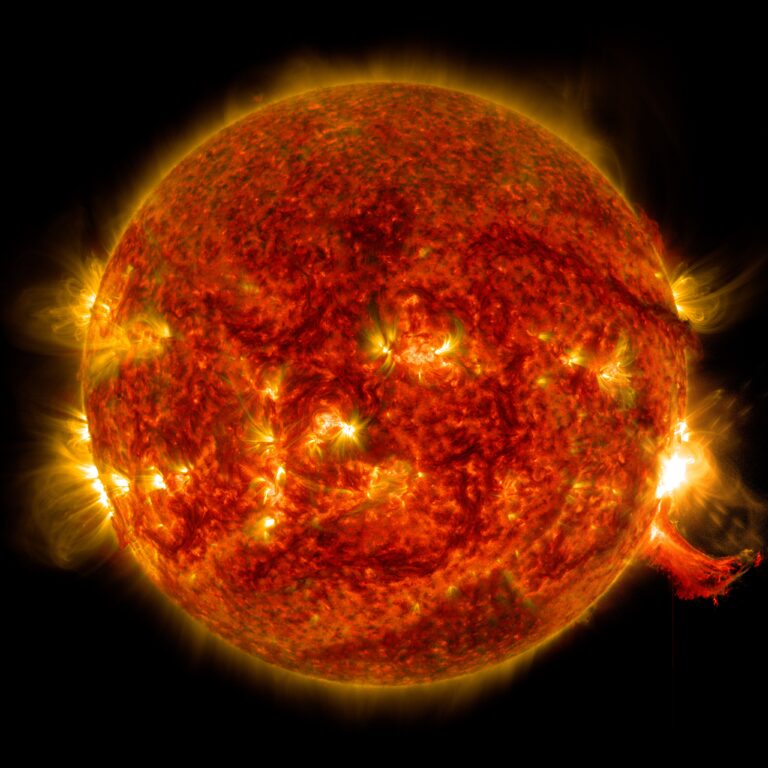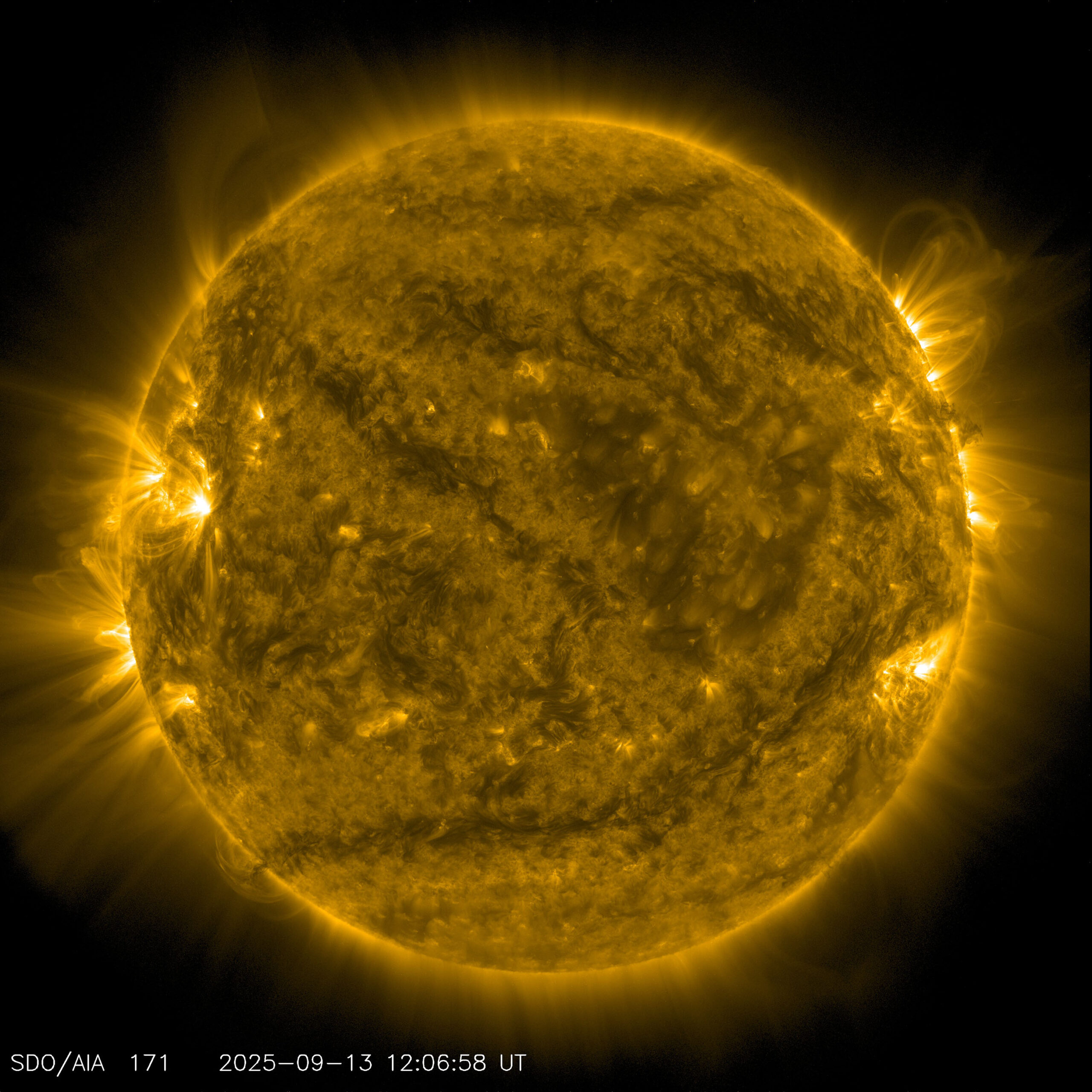
Since the Sun is so hot, how can it contain oxygen, carbon, and other elements without destroying them?
Bob Found
Indian Harbour, Nova Scotia
If you were to take a random blob of gas and heat it to solar temperatures (roughly 10,000 degrees Fahrenheit [5,500 degrees Celsius] at the surface or over 27 million F [15 million C] in the core), two things would happen. First, the gas would ionize, meaning that the electrons would be ripped away from the atoms. Second, the gas would expand. A lot. So much so that it would essentially explode.
But the Sun isn’t just a random blob of gas. It’s a really heavy random blob of gas (as are all stars). The gravity at the Sun’s surface is about 28 times stronger than Earth’s. That makes it really, really hard for the Sun to lose material. In fact, it’s the tug-of-war between gravity, which wants to pull material inward, and radiation, which wants to push material outward, that allows the Sun to maintain equilibrium and keep producing energy for billions of years.
The Sun is mostly hydrogen, which makes up around 70 percent of its mass. Then comes helium, totaling 28 percent of the Sun’s composition. The remaining 2 percent is everything else: about 1 percent oxygen, 0.3 percent carbon, and a smattering of even heavier elements.
The Sun does lose material in the form of solar wind. Twisted-up magnetic fields can give subatomic particles enough energy to escape the Sun’s enormous gravity. The solar wind carries away about 1 million to 2 million tons of mass every second, which adds up to the mass of Earth every 150 million years. But the Sun weighs more than 333,000 times Earth, so it’s got plenty of mass to spare.
Paul Sutter
Cosmologist, Johns Hopkins University, Baltimore, Maryland