But while it’s true we are star stuff, that’s only half of the story.
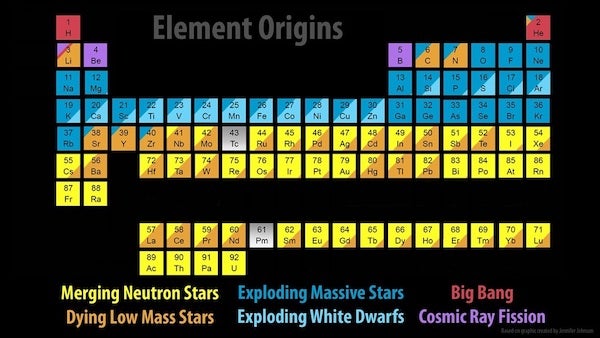
Some of the elements we know, like carbon and oxygen, are made in stars, but others — including all hydrogen, most helium, and a bit of lithium — were forged nearly 14 billion years ago through a process called Big Bang nucleosynthesis. It wasn’t until the first stars formed some 100 million years after the Big Bang that the universe began to diversify its element portfolio. And our understanding of exactly how it does that is only just being finalized.
Cosmic alchemy
The idea that smaller, lighter pieces of matter could be forged into heavier ones dates back to at least the ancient Greeks. But it wasn’t until the 1920s that scientists really began to understand the specifics.
At the time, scientists were trying to figure out what powered the Sun. Astronomer Arthur Eddington first theorized that hydrogen atoms — the lightest element — could be squeezed together under immense temperature and pressure into helium — the second lightest element. This process, known as nuclear fusion, releases colossal amounts of energy.
Over the following decades, scientists verified the mechanism, working out many of the details. And by the mid-20th century, astronomers had a good handle on how stars made elements lighter than iron.
They deduced that during the prime years of their lives, stars steadily churn hydrogen into helium within their cores. And if a star is larger than about half the mass of the Sun, once it runs out of hydrogen in its core, it begins to collapse under its own uncontested gravity. This creates additional pressure in the star’s core, which sparks helium burning and can ultimately produce by-products such as carbon and oxygen. Stars more than twice the mass of the Sun that also have carbon and oxygen from their forbearers can produce nitrogen as well.
Stars up to roughly eight times the mass of the Sun eventually reach a phase of their lives known as the asymptotic giant branch. This is where their cores become inactive, and helium and hydrogen burning migrates to the stars’ outer layers. At this stage, the stars begin the slow neutron-capture process. Also known as the s-process, this occurs in helium burning shells around stellar cores, which creates heavier elements like strontium, lead, and others.
Eventually, these relatively low-mass stars collapse into dense objects known as white dwarfs. And as they do, they can expel supersonic winds, releasing shells of gas that create beautiful — albeit short-lived — planetary nebulae. This liberates the stars’ elemental creations into interstellar space, where some of the enriched material will be recycled into new stars and planetary systems.
Explosive origins
However, when a more massive star (greater than about eight solar masses) reaches the end of its life, it can explode as a core-collapse supernova. Such supernovae can leave behind neutron stars that produce highly neutron-rich winds.
In these winds, additional elements are formed through the rapid neutron-capture process, or r-process. When the nuclei of existing atoms capture extra free neutrons, the resulting product can be radioactive — meaning it will decay into a different version of itself or a new element entirely — or it can remain stable.
The r-process is very similar to the s-process, except it’s much quicker. The s-process can take decades or centuries to capture successive neutrons, with the entire elemental transformation taking tens of thousands of years. However, a supernova can produce roughly a billion billion billion neutrons per cubic inch, so the r-process is nearly instantaneous — at least in astronomical terms. For example, through the r-process, an iron atom can be transformed into uranium in less than a second.
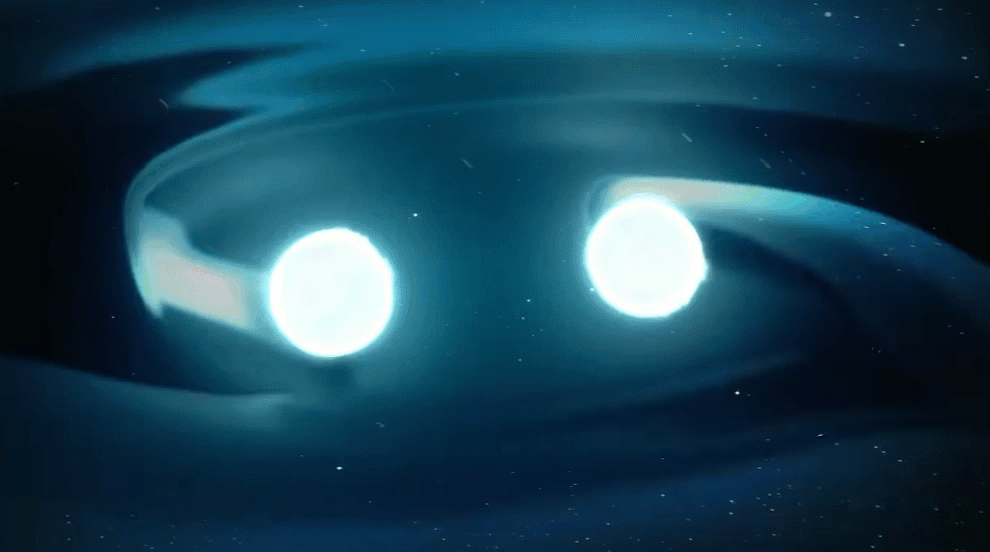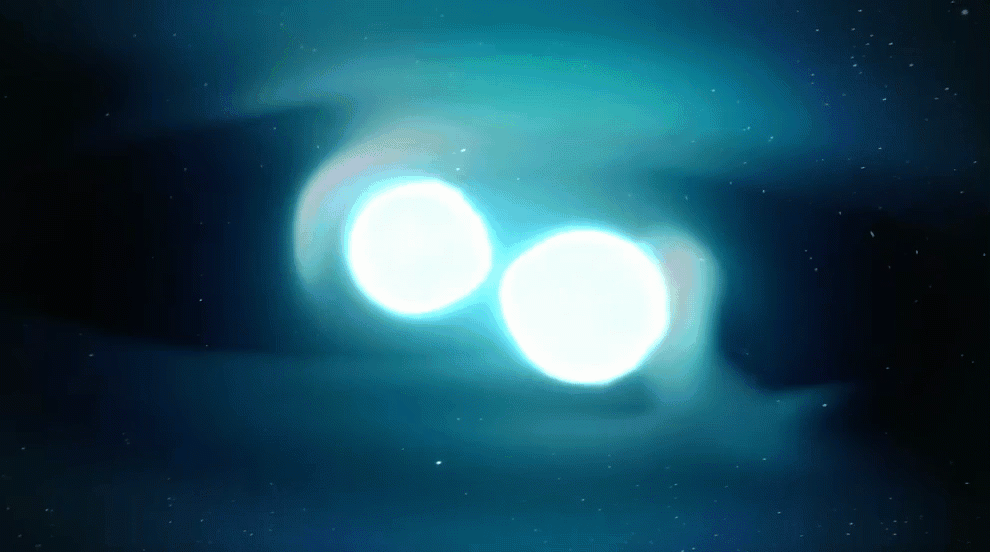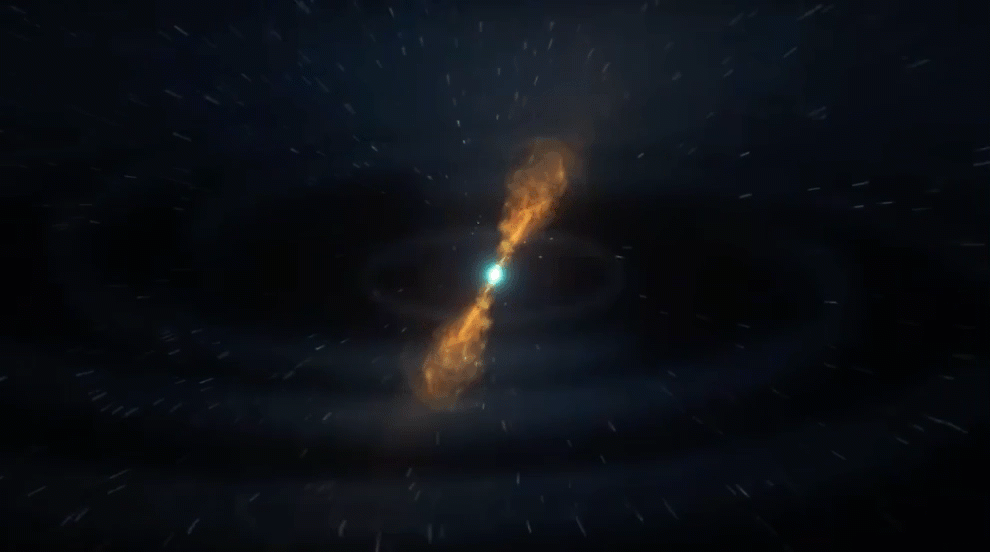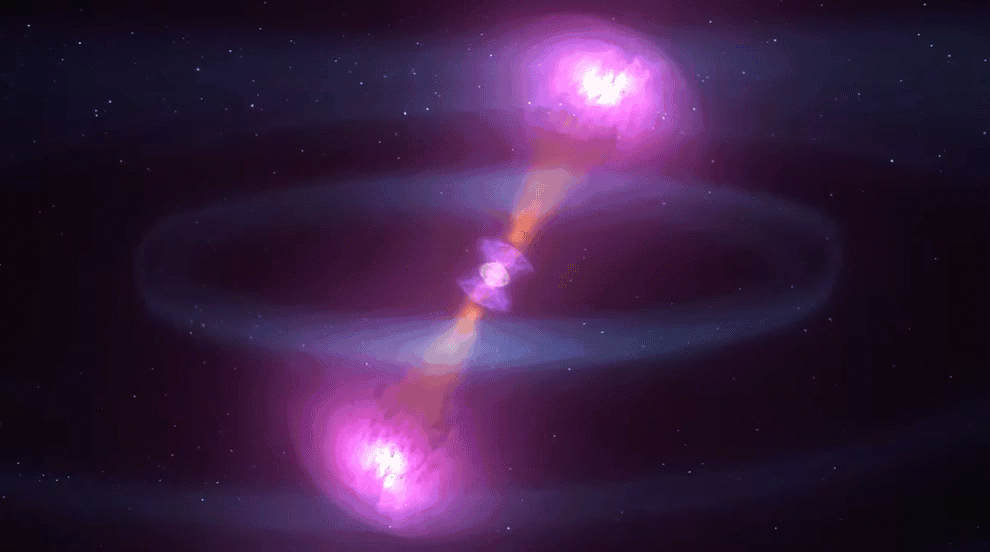
Astronomers also recently confirmed another suspected r-process site: merging neutron stars.
Signatures of elements that are only created by the r-process were observed coming from the location of a confirmed neutron star merger picked up by gravitational waves. Even though such mergers are rarer than supernovae, astronomers now think that neutron star mergers are the primary sites of most heavy r-process elements. After all, this observed gravitational-wave event alone is expected to have produced an estimated three to 13 Earth-masses worth of gold.
Now that astronomers know how the universe forges all (or at least most) of its elements, the next step is working to understand exactly how much of each element is produced through various processes, as well as where they tend to occur. By building on this knowledge, researchers ultimately hope it will allow them to easily probe the complex history of any galaxy by simply looking at the ratios of its elements.